KEY POINTS
- Fructose was originally a seasonal natural nutrient, mainly consumed in summer and fall in fruits and vegetables. In the industrial era, it became a permanent constituent of our diet, essentially a constituent of added sugars (sucrose, high-fructose corn syrup).
- Fructose cannot be directly metabolized by most cells in our body. It has to be processed first in the gut, liver and kidneys, where it is converted into glucose, lactate and fatty acids.
- Too much dietary fructose along with excess energy intake and low physical activity can cause hepatic insulin resistance, hypertriglyceridemia and increased hepatic fat content.
- In exercising athletes, net carbohydrate oxidation increases with glucose ingestion in a dose-dependent manner until a plateau is reached at about 1g/min. The addition of fructose to glucose drinks can further increase carbohydrate oxidation.
- During exercise, substantial amounts of fructose can be converted into lactate in splanchnic organs if available and released in the systemic circulation to be oxidized in contracting muscles. This “reverse fructose-lactate Cori cycle” provides additional energy substrate to muscle during exercise.
- Conversion of fructose into glucose and lactate in splanchnic organs is associated with enhanced splanchnic energy expenditure, while muscle energy efficiency is minimally altered.
- During recovery after exercise, glucose and fructose mutually enhance their gut absorption and their storage as glycogen in the liver.
INTRODUCTION
Fructose is naturally present in fruits and vegetables either in its monosaccharide form (“free” fructose), or as part of sucrose, a disaccharide formed of one molecule of glucose linked to one molecule of fructose. Glucose, sucrose and fructose activate sweet taste receptors located on the tongue, which comes with an activation of the dopaminergic reward pathways located in the meso-limbic nervous system (Lenoir et al., 2007). The sensation elicited by sweet foods is therefore felt as pleasant, and favors the consumption of sugary foods.
In early hominids, fructose consumption from fruit, berries and honey may have been substantial (reviewed in Tappy & Rosset, 2017). However, at that time, fructose intake likely fluctuated with geographical migrations and seasonal rhythmicity. In the Neolithic era, with the settlement as farmers, cereals became the main food component and the most prevalent carbohydrate source was starch, a glucose polymer. Sugar remained a rare, very expensive product until the development of European colonies in Asia and the Americas. Its consumption has however become continuous in the industrial era. In 1997, total individual fructose consumption averaged 60 g/day in the USA. Of this, roughly 75% was from sugar-sweetened beverages and industrial foods (cereal products, confectionary, candies) to which sugar had been added, and only 25% was from fresh fruits and vegetables (Marriott et al., 2009; 2010). Thus, the major part of our dietary fructose usually comes from refined sugar (sucrose) and in North America, high fructose corn syrup (HFCS) which is an industrial mixture of free glucose and fructose comprising 42-55% fructose. However, the total consumption of sugars in the USA, including fructose, decreased by ~25% in the nine years between 1999-2000 and 2008-2009 (Welsh et al., 2011).
FRUCTOSE METABOLISM IN THE LIVER
Glucose represents the preferred substrate for eukaryote cells, and can be used as an energy source by all cells of the human organism. Due to the need for conserving energy between meals, and the fact that fat is more compact and lighter than carbohydrate as an energy storage form, most human cells (with the exception of the brain) have evolved to rely on glucose in the hours after meals, and on fatty acids otherwise.
In contrast, fructose cannot be directly metabolized in most cells of our organism. Instead, it undergoes a first step processing in the liver through a pathway known as “fructolysis.” This pathway involves specific fructose-metabolizing enzymes: 1) fructokinase, which catalyzes the synthesis of fructose-1-phosphate (F-1-P); 2) aldolase B, which catalyzes its degradation into glyceraldehyde and dihydroxyacetone-phosphate (DHAP); and 3) triokinase, which converts glyceraldehyde into glyceraldehyde-3-phosphate (GAP) (Van den Berghe, 1994). The end-products of fructolysis, GAP and DHAP are also intermediates of glycolysis and hence further metabolic steps are shared with glucose metabolism.
When glucose is used as an energy substrate in the liver or in any cell type of the organism, glycolysis is tightly regulated to match cellular energy demand. This is attained by an inhibition of phosphofructokinase (the enzyme converting fructose-6-phosphate into fructose 1,6-bisphosphate in the glycolytic pathway) by intracellular ATP and citrate levels. In contrast, when fructose is metabolized in hepatocytes, there is no negative feedback on fructolysis enzymes, and all fructose molecules are completely converted into triose-phosphates, which are then further processed into acetyl-CoA, lactate, glucose, and eventually fatty acids and triglycerides (Mayes, 1976).
The relative proportion of fructose metabolized to each of these end-products has been generally evaluated in isotope studies. In resting subjects, 30-50% of ingested fructose was secreted into the circulation as glucose and 10-15% was stored as hepatic glycogen in the 4-6 h post ingestion (Sun & Empie, 2012). In addition, some 25% was released into the circulation as lactate (Jandrain et al., 1993). Finally a minor portion (~1-10%) of fructose can be converted into fatty acids and triglycerides (TG) in the metabolic pathway known as “de novo lipogenesis” (Sun & Empie, 2012).
FRUCTOSE METABOLISM IN KIDNEY PROXIMAL TUBULE CELLS AND ENTEROCYTES
While it is generally assumed, for simplification, that fructose is metabolized in the liver, it has been long known that renal proximal tubule cells also express fructolytic enzymes. The functional significance and possible pathological dysfunctions of kidney fructose metabolism still remain largely unexplored. Circulating fructose concentrations generally do not exceed 0.6 mmol/L after meals, but can increase up to 1-3 mmol/L with intravenous fructose infusion. Under such conditions, the kidneys contribute 20% of the total fructose metabolism (Björkman & Felig, 1982; Björkman et al., 1989).
Besides hepatocytes and kidney proximal tubule cells, small bowel enterocytes also express the complete enzymatic machinery required for fructose metabolism (Haidari et al., 2002; Rajas et al., 1999). Enterocytes thus contribute to overall gluconeogenesis from fructose and endogenous glucose production, as well as to de novo lipogenesis and secretion of TG rich lipoprotein particles. However, the local function of these pathways in enterocytes, and the relative contribution of the gut to overall fructose metabolism, remains speculative. One hypothesis is that intracellular fructose metabolism may be instrumental in promoting gut fructose absorption. Unlike glucose, which is mostly absorbed through a secondary active sodium-glucose co-transporter (SGLT1), fructose enters the enterocytes through GLUT5-mediated facilitated diffusion (Douard & Ferraris, 2013).
EFFECT OF FRUCTOSE CONSUMPTION IN HUMANS
In healthy subjects, fructose consumption is associated with increased endogenous glucose production, fasting and postprandial plasma triglyceride and lactate concentrations, and intrahepatocellular lipid concentrations. These metabolic alterations are the direct consequence of processing of fructose in fructokinase-expressing cells in the splanchnic area, and hence may be considered as normal adaptations to a fructose-rich diet. When associated with a high energy intake and low physical activity, they may however favor the development of diabetes and cardiovascular diseases. In turn, a few recent reports also indicate that early markers of these alterations can be corrected when appropriate physical activity is performed (Egli et al., 2013; Wilburn et al., 2015).
FRUCTOSE METABOLISM DURING EXERCISE
Exercise is associated with a high energy requirement by the contracting muscles. This energy can be obtained either from carbohydrate (glucose) and fat oxidation, or from anaerobic glycolysis alone (for relatively short periods of time).
Carbohydrate oxidation during exercise is partially dependent on exogenous carbohydrate intake. Glucose ingested during exercise is oxidized in a dose-dependent manner until a plateau is reached at ~1.0 g/min. It has been proposed that this limit is due to exogenous glucose absorption being maximal at these rates of glucose ingestion (Hawley et al., 1992).
Many studies have evaluated whether fructose drinks may be beneficial during exercise. Labelled (13C) fructose has been shown to be oxidized during exercise (Jandrain et al., 1993); however, pure fructose did not confer any advantage compared to glucose. In fact, adverse gastrointestinal effects secondary to incomplete gut absorption of pure fructose may be observed (Jeukendrup, 2010). Fructose, however, may have beneficial effects when administered together with glucose by increasing total gut hexoses’ absorption. Indeed, fructose enters the enterocyte through a facilitated glucose transporter GLUT5 rather than through the SGLT1 used for glucose. Several studies have documented that larger maximal total and exogenous carbohydrate oxidations were obtained with the ingestion of fructose-glucose mixtures vs. glucose alone (Jeukendrup, 2010). The increase in total carbohydrate oxidation with the addition of fructose to glucose drinks in exercising athletes (Jeukendrup, 2010) may appear surprising given the absence of fructokinase in skeletal muscle, and the fact that muscle hexokinase has a much lower affinity for fructose than glucose. It therefore appears to reflect oxidation by muscles of glucose and/or lactate synthesized from fructose in hepatocytes.
Splanchnic Fructose Conversion into Lactate and Reverse Hepatic-Muscle Cori Cycle during Exercise
There is solid experimental evidence that fructose conversion into lactate in splanchnic tissue is one significant metabolic pathway for fructose disposal. Catheterization studies in dogs have documented that the liver has a net lactate uptake in fasting conditions and after a glucose meal, but switches to a net lactate production/release after fructose administration (Donmoyer et al., 2002). It has further been postulated that up to 25% of the fructose carbons may be disposed through this pathway in resting conditions (Topping & Mayes, 1971).
One study documented the pathways used for fructose disposal in athletes fed glucose alone or glucose with 13C-fructose during submaximal exercise. When pure glucose was administered orally as repeated bolus drinks at a rate of 2.0 g/min, whole body glucose production and utilization (corresponding to the sum of endogenous glucose production and glucose having been absorbed from the gut minus hepatic glucose uptake) was estimated to be ~1.0 g/min, and was equal to whole body carbohydrate oxidation. Lactate production and utilization was about 0.6 g/min, and mainly reflected glucose-lactate shuttles between skeletal muscle fibers (Brooks, 2000). In contrast, when the subjects ingested a mixture of 0.8 g/min 13C-fructose + 1.2 g glucose/min instead of pure glucose, both glucose and lactate production increased significantly, and total carbohydrate oxidation further increased by 30%. About half of this increase was accounted for by lactate oxidation. This contrasts with the general concept that lactate is mainly produced by skeletal muscle to be used by the liver to synthesize glucose (Cori cycle) (Reichard et al., 1963), and indicates that, during fructose metabolism, the liver actually produces lactate to be used as an energy substrate by the contracting muscles.
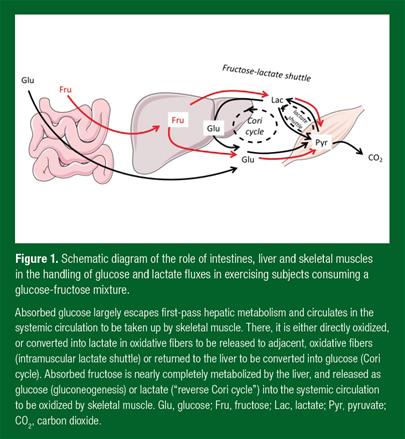
Energetics of Fructose and Glucose during Exercise
Replacing glucose with fructose as a dietary energy source during exercise has some consequences on muscle energy efficiency (Tappy et al., 2013) (Fig. 1). Glucose is taken up by contacting skeletal muscles and results in the total synthesis of 29.5 ATP. Overall, the oxidation of 1 molecule of plasma glucose uses 6 molecules of oxygen (O2) and 2 ATP and produces 6 molecules of carbon dioxide (CO2) and 29.5 ATP, corresponding to 27.5 ATP gained in working muscle, i.e., 4.58 ATP/O2 molecule.
In comparison, ATP, O2 and CO2 fluxes slightly vary when fructose is first metabolized in the liver to be secondarily oxidized in muscle (Tappy et al., 2013). When fructose is converted into glucose in the liver it consumes 2 ATP. When this newly synthesized glucose is subsequently oxidized in skeletal muscle, the overall metabolic pathway uses 6 O2 and 4 ATP and produces 6 CO2 and 29.5 ATP for each fructose molecule, representing a net gain of 25.5 ATP, or 4.25 ATP/oxygen. Interestingly, the energy yield in skeletal muscle is identical to that of glucose, but there is additional energy expended in the liver.
When fructose is converted into lactate, which is subsequently oxidized in contracting muscle, the overall metabolic process uses 6 O2 and 2 ATP and produces 6 CO2 and 29.5 ATP as with direct oxidation. In the liver, however, fructolysis consumes 2 ATP and conversion to pyruvate produces 4 ATP, resulting in 2 ATP gained. In contrast, in skeletal muscle, 2 lactates are transported into the cells through facilitated diffusion, and their complete mitochondrial oxidation requires 6 O2 and produces 25.5 ATP, corresponding to 4.25 ATP/oxygen.
In summary, the energy efficiency for fructose oxidation in muscle is somewhat lower than for dietary glucose or starch oxidation. However, hepatic fructolysis into lactate may provide a substantial energy supply to the working muscle when the glycolysis rate is limiting.
EFFECTS OF EXOGENOUS FRUCTOSE DURING RECOVERY
After exercise, athletes face a second metabolic challenge, i.e., they have to restore their initial muscle and liver energy or glycogen stores. This is a major issue for athletes involved in repeated strenuous activity, such as multi-day cycling or running races, or playing games on subsequent days, as well as in general training. In these cases, the recovery of energy stores plays a fundamental role in performance and training adaptation.
It has been well documented that both hepatic and muscle glycogen synthesis can occur after ingestion of fructose (Nilsson & Hultman, 1974). Some studies even suggested that postprandial hepatic glycogen synthesis was larger after ingestion of fructose than glucose (Decombaz et al., 2011). There is also limited evidence that a high fructose diet may also increase intramyocellular lipid contents (Le et al., 2009).
Interestingly, whether in fruits or honey, in refined cane or beet sugar, or in high fructose corn syrup, fructose is always associated with roughly equivalent amounts of glucose. These two hexoses mutually facilitate their metabolism in splanchnic tissues. Indeed, the maximal rate of absorption of pure fructose is limited in many individuals, and partial malabsorption, with or without gastrointestinal symptoms, is frequently observed after ingestion of large fructose loads (Latulippe & Skoog, 2011). Co-ingestion of glucose together with fructose enhances gut fructose absorption and prevents malabsorption (Latulippe & Skoog, 2011). In the liver, the fructolytic intermediate fructose-1-phosphate interacts with a glucokinase-regulatory protein, and thus activates glucokinase and enhances hepatic glycogen synthesis. Interestingly, the F-1-P concentrations needed to exert this effect are very low, and hence this can be observed with small “catalytic” doses of fructose added to a glucose meal (Moore et al., 2012). Recent studies have demonstrated that sucrose ingestion following exhaustive exercise improves liver glycogen resynthesis, compared to glucose alone (Fuchs et al., 2016; Gonzalez et al., 2017).
PRACTICAL APPLICATIONS
- Fructose can constitute a substantial source of energy in the human diet. It is obviously a dispensable nutrient, and no adverse effects of a fructose-deprived diet have been reported.
- Excess fructose intake can cause dyslipidemia, hepatic fat storage and hepatic insulin-resistance in sedentary subjects.
- There is no risk of ingesting reasonable amounts of fructose when performing physical activity, and exercise attenuates the possibly deleterious effects of fructose overfeeding.
- While glucose and fatty acids remain the primary energy substrates for contracting skeletal muscle, fructose presents some potential advantages for individuals involved in strenuous physical exercise by sustaining hepatic glucose production and providing extra energy as lactate to contracting skeletal muscle.
- During exercise, fructose can be conveniently provided in energy-rich solid or liquid foods that are easily consumed. Compared to glucose, it elicits a minimal glycemic response. Fructose is absorbed by different intestinal transporters than glucose absorption, which may allow it to increase muscle energy provision when glucose utilization is maximally stimulated.
- In the recovery period, fructose administered together with glucose may be a convenient, well tolerated method of increasing total carbohydrate absorption and total energy intake, to enhance hepatic energy uptake and glycogen synthesis.
SUMMARY
Fructose is a hexose, which is naturally present in fruits, honey, sucrose and high fructose corn syrup. In contrast to glucose, fructose cannot be directly metabolized by most cells of our body, and needs first to be converted into lactate, glucose or fatty acids. This processing of fructose carbons is performed by enterocytes, hepatocytes and kidney proximal tubule cells, which all express specific fructose metabolizing enzymes. In healthy subjects, fructose overfeeding is associated with an increased endogenous glucose production, increased fasting and postprandial plasma triglyceride concentrations, hyperlactatemia, and an increase in intrahepatocellular lipid concentrations. When associated with a high energy intake and low physical activity, these metabolic alterations may favor the development of diabetes and cardiovascular diseases. When fructose is ingested during exercise, splanchnic lactate production and oxidation of lactate in skeletal muscle constitutes a physiological pathway for the use of fructose energy. During recovery after exercise, co-ingestion of glucose and fructose can increase total digestive carbohydrate absorption and may enhance glycogen repletion.
REFERENCES
Björkman, O., and P. Felig (1982). Role of the kidney in the metabolism of fructose in 60-hour fasted humans. Diabetes 31:516-520.
Björkman, O., R. Gunnarsson, E. Hagström, P. Felig, and J. Wahren (1989). Splanchnic and renal exchange of infused fructose in insulin-deficient type 1 diabetic patients and healthy controls. J. Clin. Invest. 83:52-59.
Brooks, G.A. (2000). Intra- and extra-cellular lactate shuttles. Med. Sci. Sports Exerc. 32:790-799.
Decombaz, J., R. Jentjens, M. Ith, E. Scheurer, T. Buehler, A. Jeukendrup, and C. Boesch (2011). Fructose and galactose enhance postexercise human liver glycogen synthesis. Med. Sci. Sports Exerc. 43:1964-1971.
Donmoyer, C.M., D.B. Lacy, Y. Zhang, S.S. Chen, and O.P. McGuinness (2002). Impact of chronic fructose infusion on hepatic metabolism during TPN administration. Am. J. Physiol. 283:E1151-E1158.
Douard, V., and R.P. Ferraris (2013). The role of fructose transporters in diseases linked to excessive fructose intake. J. Physiol. 591:401-414.
Egli, L., V. Lecoultre, F. Theytaz, V. Campos, L. Hodson, P. Schneiter, B. Mittendorfer, B.W. Patterson, B.A. Fielding, P. Gerber, V. Giusti, K. Berneis, and L. Tappy (2013). Exercise prevents fructose-induced hypertriglyceridemia in healthy young subjects. Diabetes 62:2259-2265.
Fuchs, C.J., J.T. Gonzalez, M. Beelen, N.M. Cermak, F.E. Smith, P.E. Thelwall, R. Taylor, M.I. Trenell, E.J. Stevenson, and L.J. van Loon (2016). Sucrose ingestion after exhaustive exercise accelerates liver, but not muscle glycogen repletion compared with glucoe ingestion in trained athletes. J. Appl. Physiol. 120:1328-1334.
Gonzalez, J.T., C.J. Fuchs, J.A, Betts, and L.J. van Loon (2017). Glucose plus fructose ingestion for post-exercise recovery - greater than the sum of its parts? Nutrients 9:E344.
Haidari, M., N. Leung, F. Mahbub, K.D. Uffelman, R. Kohen-Avramoglu, G.F. Lewis, and K. Adeli (2002). Fasting and postprandial overproduction of intestinally derived lipoproteins in an animal model of insulin resistance. Evidence that chronic fructose feeding in the hamster is accompanied by enhanced intestinal de novo lipogenesis and ApoB48-containing lipoprotein overproduction. J. Biol. Chem. 277: 31646-31655.
Hawley, J.A., S.C. Dennis, and T.D. Noakes (1992). Oxidation of carbohydrate ingested during prolonged endurance exercise. Sports Med. 14:27-42.
Jandrain, B.J., N. Pallikarakis, S. Normand, F. Pirnay, M. Lacroix, F. Mosora, C. Pachiaudi, J.F. Gautier, A.J. Scheen, J.P. Riou, and P.J. Lefebvre (1993). Fructose utilization during exercise in men: rapid conversion of ingested fructose to circulating glucose. J. Appl. Physiol. 74:2146-2154.
Jeukendrup, A.E. (2010). Carbohydrate and exercise performance: the role of multiple transportable carbohydrates. Curr. Opin. Clin. Nutr. Metab. Care 13:452-457.
Latulippe, M.E., and S.M. Skoog (2011). Fructose malabsorption and intolerance: effects of fructose with and without simultaneous glucose ingestion. Crit. Rev. Food Sci. Nutr. 51:583-592.
Le, K.A., M. Ith, R. Kreis, D. Faeh, M. Bortolotti, C. Tran, C. Boesch, and L. Tappy (2009). Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Amer. J. Clin. Nutr. 89:1760-1765.
Lenoir, M., F. Serre, L. Cantin, and S.H. Ahmed (2007). Intense sweetness surpasses cocaine reward. PLoS One 2:e698.
Marriott, B.P., N. Cole, and E. Lee (2009). National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J. Nutr. 139:1228S-1235S.
Marriott, B.P., L. Olsho, L. Hadden, and P. Connor (2010). Intake of added sugars and selected nutrients in the United States, National Health and Nutrition Examination Survey (NHANES) 2003-2006. Crit. Rev. Food Sci. Nutr. 50:228-258.
Mayes, P.A. (1976). Control of hepatic triacylglycerol metabolism. Biochem. Soc. Trans. 4:575-580.
Moore, M.C., K.C. Coate, J.J. Winnick, Z. An, and A.D. Cherrington (2012). Regulation of hepatic glucose uptake and storage in vivo. Adv. Nutr. 3:286-294.
Nilsson, L.H., and E. Hultman (1974). Liver and muscle glycogen in man after glucose and fructose infusion. Scand. J. Clin. Lab. Invest. 33:5-10.
Rajas, F., N. Bruni, S. Montano, C. Zitoun, and G. Mithieux (1999). The glucose-6 phosphatase gene is expressed in human and rat small intestine: regulation of expression in fasted and diabetic rats. Gastroenterology 117:132-139.
Reichard, G.A.J., N.F. Moury, N.J. Hochella, A.L. Patterson, and S. Weinhouse (1963). Quantitative estimation of the Cori cycle in the human. J. Biol. Chem. 238:495-501.
Sun, S.Z., and M.W. Empie (2012). Fructose metabolism in humans - what isotopic tracer studies tell us. Nutr. Metab. 9:89.
Tappy, L., L. Egli, V. Lecoultre, and P. Schneider (2013). Effects of fructose-containing caloric sweeteners on resting energy expenditure and energy efficiency: a review of human trials. Nutr. Metab. 10:54.
Tappy, L., and R. Rosset (2017). Fructose metabolism from a functional perspective: implications for athletes. Sports Med. 47:S23-S32.
Topping, D.L., and P.A. Mayes (1971). The concentration of fructose, glucose and lactate in the splanchnic blood vessels of rats absorbing fructose. Nutr. Metab. 13:331-338.
Van den Berghe, G. (1994). Inborn errors of fructose metabolism. Ann. Rev. Nutr. 14:41-58.
Welsh, J.A., A.J. Sharma, L. Grellinger, and M.B. Vos (2011). Consumption of added sugars is decreasing in the United States. Am. J. Clin. Nutr. 94:26-34.
Wilburn, J.R., J. Bourquin, A. Wysong, and C.L. Melby (2015). Resistance exercise attenuates high-fructose, high-fat-induced postprandial lipemia. Nutr. Metab. Insights 8:29-35.